IMPORTANT SAFETY INFORMATION
What is UPNEEQ?
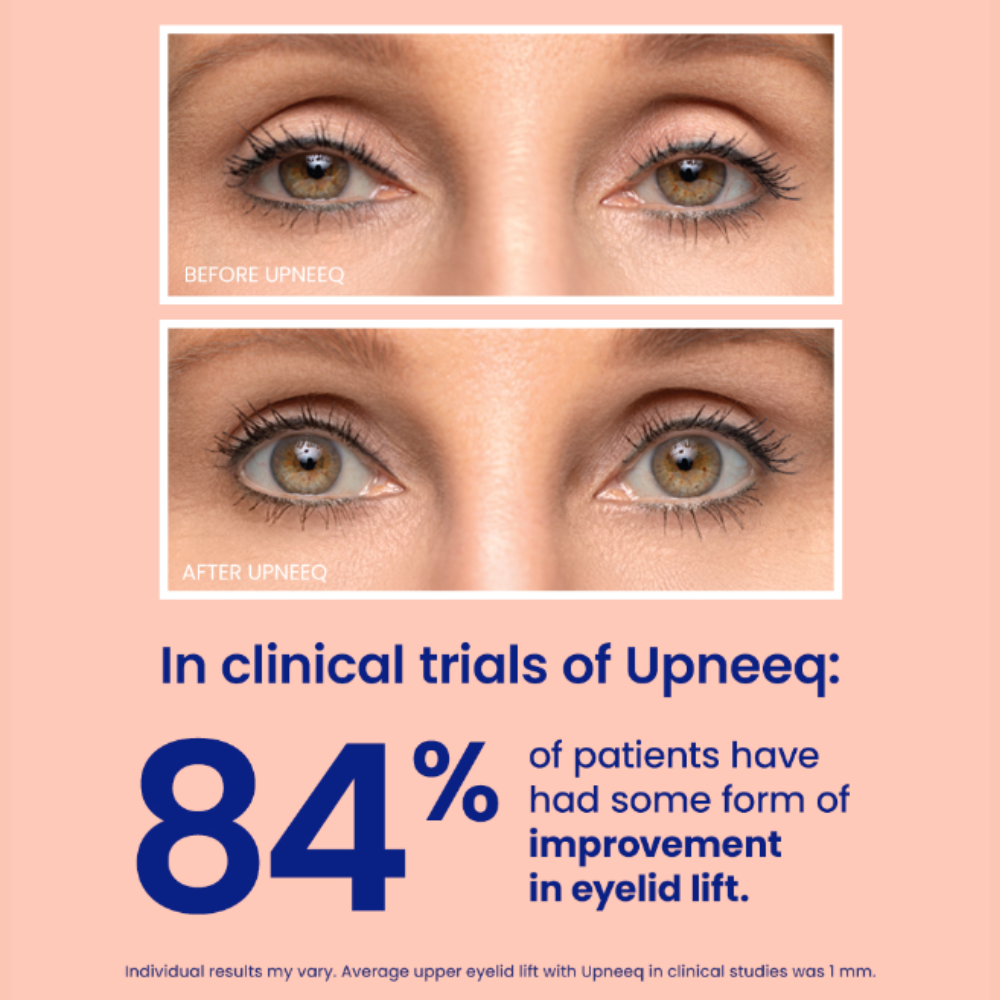
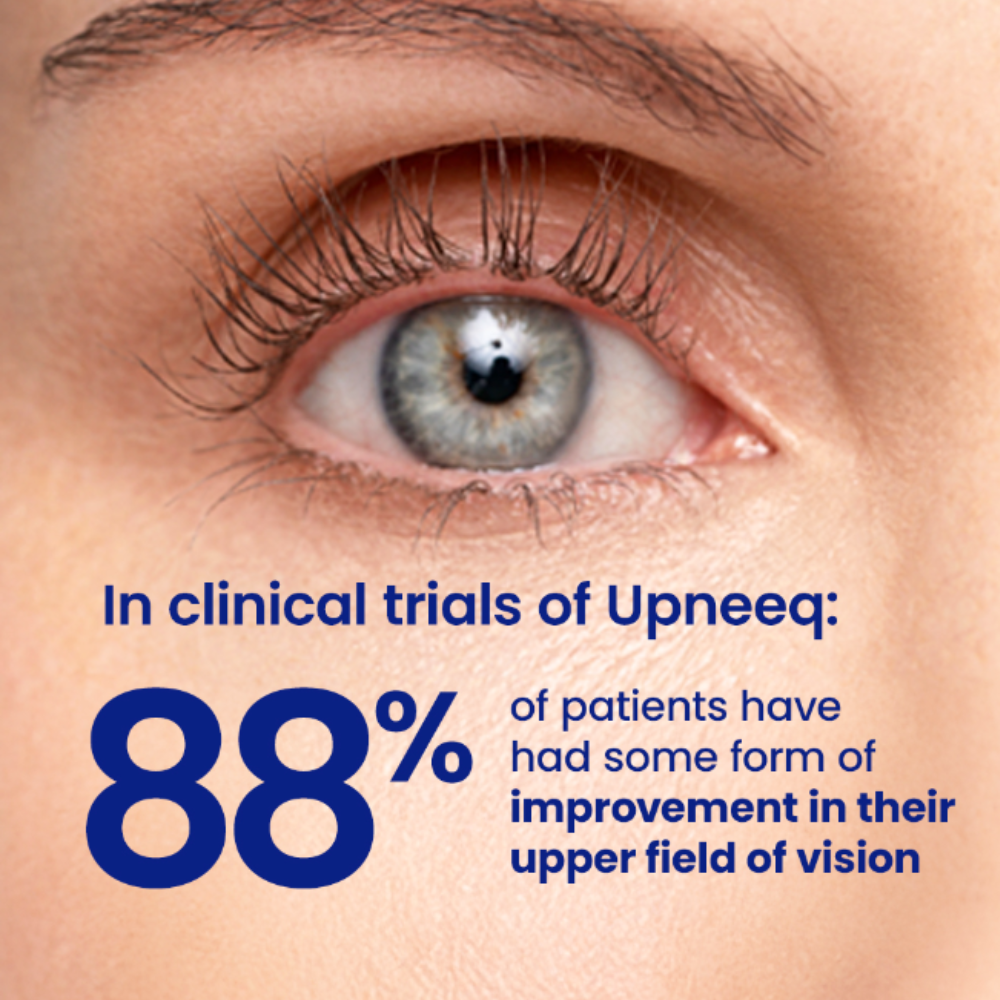
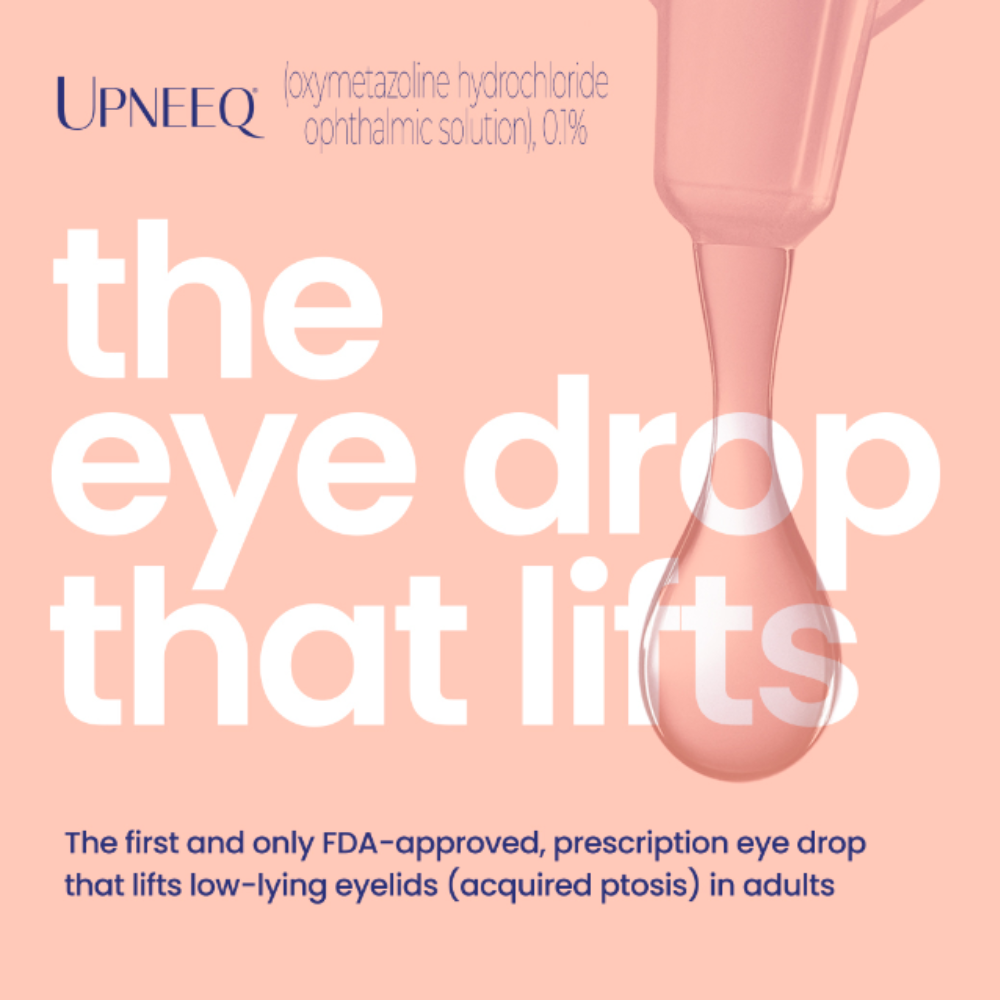
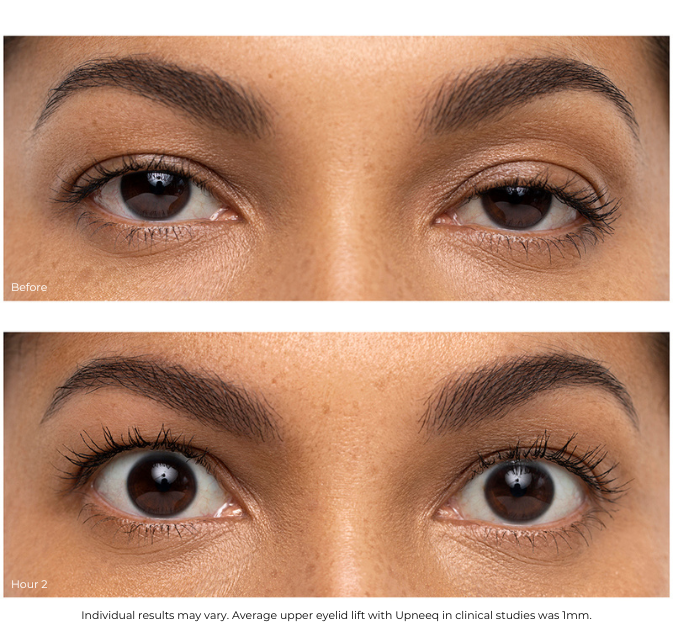
UPNEEQ ® (oxymetazoline hydrochloride ophthalmic solution), 0.1% is a prescription eye drop used to treat acquired blepharoptosis (low-lying lids) in adults.
What warnings and precautions are associated with UPNEEQ?
- Low-lying lids may be related to conditions such as stroke and/or brain aneurysm, Horner syndrome, myasthenia gravis, loss of the ability to move eye muscles, eye infection and eye tumors. Tell your doctor if you have any of these conditions.
- UPNEEQ is a type of medication that may affect your blood pressure. If you have heart disease, uncontrolled high or low blood pressure, or feel faint at rest or when quickly standing up, you should call your doctor if your symptoms get worse.
- Patients with reduced blood flow to the brain or heart, or patients who experience eye or mouth dryness due to an immune system disorder (Sjögren’s syndrome), should use care when taking UPNEEQ. Call your doctor immediately if you feel your symptoms may be getting worse.
- UPNEEQ may increase the risk of eye pressure due to fluid buildup (angle-closure glaucoma) in patients with untreated narrow-angle glaucoma. Call your doctor immediately if you feel increased pressure in your eye after using UPNEEQ.
- Do not let the tip of the UPNEEQ vial touch your eye or any other surface. This can help prevent eye injury or contamination. Each UPNEEQ vial is for one-time use and should be discarded after being used.
What are the most common side effects of UPNEEQ?
The most common adverse reactions with UPNEEQ (occurring in 1-5% of patients) were eye inflammation, eye redness, dry eye, blurred vision, eye pain at time of use, eye irritation, and headache.
What should my doctor know about before prescribing me UPNEEQ?
- Your doctor should review your full medical history before prescribing UPNEEQ.
- UPNEEQ belongs to a class of medication (alpha-adrenergic agonists) that may affect your blood pressure. Use UPNEEQ carefully if you currently take an alpha-adrenergic agonist medication to treat heart disease or an enlarged prostate. Patients taking beta-blockers, or other medications to treat hypertension or an abnormal heartbeat, should also be careful when using UPNEEQ.
- Patients who use a certain class of antidepressant medication (monoamine oxidase inhibitors) should also be careful when using UPNEEQ, as it may affect the way your body absorbs the medication.
These are not all of the possible side effects of UPNEEQ. Tell your doctor if you have any side effect that bothers you or does not go away. Call your doctor for medical advice about side effects.
To report side effects or product complaints, contact RVL Pharmaceuticals at 1-877-482-3788. You may also report side effects to the FDA by calling 1-800-FDA-1088 or visit www.fda.gov/medwatch.
This is a summary of the most important safety information for UPNEEQ. For more in-depth safety information, please review the full Prescribing Information for UPNEEQ.